Next: Identification of Close-Packed Structures by X-ray Up: Close-Packed Structures Previous: (ii) Inorganic compounds
![[IUCr Home Page]](/iucr-top/logos/iucrhome.gif)
![[Commission Home Page]](/iucr-top/logos/cteach.gif)
![]()
![]()
![]()
Next: Identification of Close-Packed Structures by X-ray
Up: Close-Packed Structures
Previous: (ii) Inorganic compounds
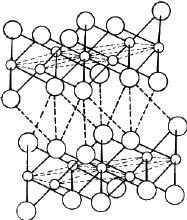
Cadmium iodide is an ionic compound, the ionic radii of Cd and I being 0.97
Å and 2.16 Å respectively2. The structure consists of a close-
packing of the I ions with the Cd ions distributed among the octahedral voids.
The radius ratio r Cd/r I = 0.45 permits the Cd ions to
occupy the octahedral voids. Since there are only half as many Cd ions as I
ions in the structure, only half of the total octahedral voids are occupied.
Thus the Cd and I layers are not stacked alternately; there is one Cd layer
after every two I layers as shown in Fig. 12. The structure therefore consists
of molecular sheets (called minimal sandwiches) with a layer of Cd ions
sandwiched between two close-packed layers of I ions. The binding within the
minimal sandwich is ionic in character and is much stronger than the binding
between successive sandwiches which is of van der Waals type. It is because of
the weak van der Waals bonding between the successive minimal sandwiches that
the material possesses the easy cleavage characteristic of a layer structure.
Cadmium iodide structures can have a centre of symmetry in octahedral voids, but
cannot have a symmetry plane perpendicular to [00.1]. Cadmium iodide can
therefore have five possible space groups - P 3m 1,
P ![]() m , R 3m ,
R
m , R 3m ,
R ![]() m and P 63mc . Cubic symmetry is
not possible in CdI2 on account of the presence of Cd atoms. The most
common modifications of CdI2 are 4H and 2H with stacking
sequences /A
m and P 63mc . Cubic symmetry is
not possible in CdI2 on account of the presence of Cd atoms. The most
common modifications of CdI2 are 4H and 2H with stacking
sequences /A![]() B C
B C![]() B/...., and /A
B/...., and /A![]() B/A
B/A![]() B/....
respectively, where the Greek letters denote the positions of Cd ions. In
addition, this material also displays2,19 a number of polytype
modifications of large repeat periods. From the structure of CdI2 it
follows that the identity period of all such modifications must consist of an
even number of iodine layers. The h/a ratio in all these modifications
of CdI2 is 0.805 which differs considerably from the ideal value of 0.8165.
B/....
respectively, where the Greek letters denote the positions of Cd ions. In
addition, this material also displays2,19 a number of polytype
modifications of large repeat periods. From the structure of CdI2 it
follows that the identity period of all such modifications must consist of an
even number of iodine layers. The h/a ratio in all these modifications
of CdI2 is 0.805 which differs considerably from the ideal value of 0.8165.
 |
Copyright © 1981, 1997 International Union of Crystallography
IUCr Webmaster